Bioanalytical testing is a cornerstone in pharmaceutical development, providing crucial data on pharmacokinetics, pharmacodynamics, and immunogenicity to determine the patient safety margins of effective new drugs. For laboratories conducting small and large molecule bioanalysis, harmonized sample management processes, study protocol organization, quantitative assay development and validation, and data flow optimization are compulsory. Adopting a combined LIMS and ELN platform transforms disconnected practices, providing better control over data and improving efficiency and compliance with regulations.
3 min read
Streamlining Bioanalytical Testing with a Unified LIMS and ELN Solution
By LabWare on Mar 14, 2024 11:01:00 AM
Topics: LIMS Bioanalysis ELN
5 min read
Unlocking Efficiency in Petroleum Laboratories
By LabWare on Mar 6, 2024 2:30:00 AM
The digital revolution is transforming industries worldwide, and the petroleum sector is no exception. A landmark event in this transformation was the recent announcement that the Egyptian General Petroleum Corporation (EGPC) and Cairo Oil Refining Company have partnered strategically with laboratory software leader LabWare. This collaboration aims to implement LabWare’s Laboratory Information Management System (LIMS) to improve EGPC and Cairo Oil Refining laboratories' operational efficiencies and data management practices.
Topics: LIMS Oil and Gas
4 min read
Optimizing Cannabis Lab Operations: A Discussion with Green Valley Analytics CEO Jonathan Ferguson
By LabWare on Feb 21, 2024 4:20:00 PM
Green Valley Analyticsis a full-service independent analytical testing laboratory fully licensed by the Massachusetts Cannabis Control Commission. The lab offers a comprehensive menu of services to support cultivators and manufacturers licensed to grow and produce medical and recreational cannabis products.In a recent conversation with the laboratory's CEO and co-founder, Jonathan Ferguson, the spotlight was on LabWare GROW, a cloud-based laboratory information management system (LIMS)purpose-built for CBD/THC testingand its instrumental role in revolutionizing cannabis laboratory workflows.
Topics: LIMS Cannabis
3 min read
LabWare Opens Korean Office LabWare,한국 사무소 개소
By LabWare on Feb 13, 2024 9:44:59 AM
SEOUL, KOREA -- LabWare Korea Co., Ltd
LabWare is excited to announce the grand opening of our latest office, situated in the heart of Seoul, Korea.
LabWare는 대한민국 서울에 새로운 사무실의 개소를 알리게 되어 매우 기쁘게 생각합니다.
This expansion marks a significant stride forward in our global objective to provide world-leading, cutting-edge laboratory informatics solutions to customers across the globe.
이번 확장은 전 세계 고객들에게 선도적인 최첨단 실험실 정보 솔루션을 제공하려는 글로벌 목표에 한 걸음 더 나아가는 중요한 진전입니다.
Extending global coverage and complementing existing Asia Pacific Offices
Accompanying the inauguration of our Seoul Office and the establishment of LabWare Korea Co., Ltd, we are delighted to introduce our esteemed Korean Team, comprising seasoned LIMS professionals who bring a wealth of experience and local expertise to the table.
서울 사무소 개소 및 LabWare Korea Co., Ltd의 설립과 함께 풍부한 경험과 현지 전문 지식을 제공하는 숙련된 LIMS 전문가로 구성된 존경받는 한국 팀을 소개하게 되어 기쁩니다.
한국 팀은 실험실 정보 솔루션 분야에서 많은 경험을 가진 전문가들로 구성되어 있으며 한국 시장에 대한 깊은 이해와 기술적 노하우는 LIMS 고객들에게 보다 우수한 서비스를 제공할 수 있게 도와줄 것입니다.
Our Korean Office and Korean Team extend our global coverage and complement our existing Asia Pacific Offices and Teams who are located in Australia (Adelaide, Brisbane, Melbourne, Sydney), China (Beijing, Chengdu, Jinan, Nanjing, Shanghai), Indonesia (Jakarta), Japan (Tokyo), Malaysia (Kuala Lumpur), New Zealand (Auckland, Tauranga), Philippines (Manila), Singapore, Taiwan (Kaohsiung, Taipei), Thailand (Bangkok) and Vietnam (Ho Chi Minh City).
한국 지사와 한국 팀은 아시아 태평양 지사 및 팀을 보완하고 글로벌 서비스 범위를 확장합니다,
아시아 태평양 지사 및 팀은 호주 (애들레이드, 브리즈번, 멜버른, 시드니), 중국 (베이징, 청두, 진안, 난징, 상하이), 인도네시아(자카르타), 일본(도쿄), 말레이시아(쿠알라룸푸르), 뉴질랜드(오클랜드, 타우랑가), 필리핀(마닐라), 싱가포르, 대만(가오슝, 타이베이), 태국(방콕), 베트남(호치민)에 위치해 있습니다.
Visit the LabWare Korea Co., Ltd Office:
Seoul, Regus Gangnam Station Centre
16F, Gangnam Bldg
396 Seocho-daero, Seocho-gu
Seoul 06619, Korea
+82 (2) 2190 3764
Email: infoAP@labware.com
Topics: LabWare
8 min read
What Cannabis Testing Laboratories Can Do After Inspection Failure
By LabWare on Feb 6, 2024 5:16:56 PM
Cannabis testing laboratories are essential in protecting the safety and quality of cannabis products. Cannabis testing helps identify and quantify the levels of cannabinoids (such as THC and CBD) and the presence of harmful contaminants like pesticides, heavy metals, mold, and bacteria, safeguarding consumers from health risks. The cannabis plant's chemical diversity, variability in cannabinoid concentrations, difficult contaminant detection, evolving industry practices, and the continuous emergence of new compounds make testing a complex endeavor.
Topics: LIMS Cannabis
11 min read
Revolutionizing Efficiency in Biopharma Lab Operations
By LabWare on Jan 24, 2024 8:41:00 AM
The Role of Quality Control in Biopharma Lab Operations
Quality control (QC) serves as the vital heartbeat within the dynamic realm of biopharmaceutical laboratories. Beyond ensuring adherence to rigorous regulatory standards, QC emerges as a central player in continually enhancing laboratory operations and the meticulous collection of essential data. This pivotal role involves maintaining compliance and contributing significantly to the overall efficiency, reliability, and quality of processes within biopharma lab operations. By upholding rigorous quality standards, QC becomes a cornerstone for fostering excellence, reliability, and precision in pursuing innovative solutions and developing safe and effective biological medicines.
Topics: BioPharma
3 min read
Körber and LabWare lead the way with seamless MES-LIMS integration in the pharma and biopharma industry
By LabWare on Nov 9, 2023 2:45:58 AM
Lüneburg, DE / Delaware, US
LabWare® has achieved the “Ready” level required to join Körber's Ecosystem of partner companies.
With that, the two companies are taking the seamless integration capabilities between LabWare’s LIMS (Laboratory Information Management System) and Körber’s PAS-X MES (Manufacturing Execution System) to a new level of cooperation.
Körber's collaboration with LabWare marks the next milestone following the launch of Körber's "PAS-X LIMS Integration" program, a new element within the Körber Ecosystem. This program offers customers improved convenience in identifying LIMS suppliers compatible with PAS-X MES. It demonstrates to customers that Körber and its partners can significantly reduce the effort and risk involved in integrating LIMS with Körber's world-leading Manufacturing Execution System PAS-X MES by using a standardized interface.
LabWare, a trusted partner to most of the top 25 global pharmaceutical companies and a worldwide leader in laboratory informatics, brings profound expertise and operational excellence to this relationship. “Our LIMS is used at more than 29,000 laboratories with more than 150,000 daily users and a billion samples tested a year,” says Mark Gonzalez, Technical Director at LabWare. As a certified partner, LabWare demonstrates seamless integration capabilities with PAS-X MES. “The successful collaboration between LabWare and Körber has already greatly benefited our shared customers over the years,” Gonzalez adds.
The integration of MES and LIMS has emerged as a pivotal component in the digital transformation journey within the pharma and biopharma industry, especially given the laboratory's critical role in the overall production and supply chain processes. Lars Hornung, Senior Principal Alliances & Technology Partners Software, Körber Business Area Pharma, elaborates: “For instance, as part of the manufacturing process, PAS-X MES triggers a sample request to the LIMS, and the LIMS communicates detailed sample data back to the MES, enabling efficient label printing for each sample. After sample collection, PAS-X MES sends a sample confirmation to LIMS, including additional information for Quality Control (QC) purposes. The laboratory conducts the tests, and LIMS manages the results, which are then transmitted back to MES for further processing. Therefore, seamless integration between these systems is vital to streamlining processes and eliminating human error.”
Topics: LIMS LabWare BioPharma
3 min read
Streamline Cannabis Compliance with a LIMS Interface to Metrc
By LabWare on Sep 28, 2023 3:49:30 PM
In the fast-evolving world of cannabis regulation, staying compliant with state-specific tracking and reporting requirements is paramount for businesses operating in the industry. One tool that has become essential for maintaining compliance is the Marijuana Enforcement Tracking Reporting Compliance (Metrc) system. Metrc is the backbone of regulatory oversight, helping track cannabis products from seed to sale. Cannabis testing laboratories can simplify and enhance compliance management by integrating their Laboratory Information Management System (LIMS) with Metrc. The benefits of a LIMS interface to Metrc and how it can help streamline cannabis compliance.
Topics: Cannabis
2 min read
New LabWare Office Opens in Wageningen, The Netherlands
By LabWare on Sep 15, 2023 10:50:11 AM
WAGENINGEN, NL -- LabWare Nederland
LabWare® branches out to Wageningen, The Netherlands, expanding its network of over 40 offices that operate across 6 continents.
The branch in Wageningen focuses on the Benelux area (Belgium, The Netherlands, and Luxembourg), starting with a team of 6 individuals that is expected to double within the next 3 years.
A strategic choice for the advancement of scientific research and innovation
The global recognition of the Netherlands as a hub for research and innovation uniquely positions it to offer strong backing to a company such as LabWare, worldwide leader in automation and data management solutions for laboratories.
Historic and multicultural city in the central Netherlands, in the province of Gelderland, Wageningen is renowned for its University, specializing in life sciences, agriculture, technical and engineering subjects. Wageningen is therefore known also as “The city of life sciences”, with a leading position in these fields, as well as in health research and development, providing companies with both a customer base and a favorable environment for experimentation and innovation with a strong focus on sustainability.
Frank Hendriks, Territory Manager Benelux at LabWare, states: “When contemplating further expanding into Europe, we thoroughly assessed numerous potential locations, and Wageningen emerged as our top choice”. The region's strategic positioning, alongside cutting-edge infrastructure, and proximity to diverse research institutions, offers an optimal setting for fostering collaboration and innovation.
LabWare’s commitment to empower cross-sectoral sustainable solutions
LabWare promotes innovation and sustainable solutions within agriculture and food production by supporting research and development.
It is indeed LabWare’s mission to deliver laboratory automation, efficiency, data integrity, and compliance by means of informatics solutions. The LabWare laboratory automation suite allows to easily manage data, optimizing workflows and collaboration – which is vital for companies striving to develop and implement sustainable technologies.
With its new premises in Wageningen, in the Agro Business Park Garden Offices – where multiple innovative health companies are also situated – LabWare Nederland connects with relevant partners and networks, perfectly aligning with the regional focus on driving innovation and sustainable solutions for global challenges.
Visit the Wageningen Office:
Agro Business Park 2
Garden Offices
6708 PW
Wageningen, Netherlands
Topics: LabWare
3 min read
LabWare Selected as Honoree of 2023 Drexel LeBow Analytics 50 Award
By LabWare on Jul 20, 2023 9:00:00 AM
WILMINGTON, Del.-- It was announced last week, LabWare® was selected as an honoree of the 2023 Drexel LeBow Analytics 50 Award. This award recognizes and honors companies that have made a significant data-driven business impact. Past recognized recipients include Pfizer, GSK, Tyson Foods, Starbucks, and Citi, among others.
“We are honored to receive this recognition,” said Darren Mahoney, Product Manager of LabWare Analytics. “The reputation this award and university hold makes us proud of our daily work. To be recognized alongside companies like FICO, Pfizer, and the United Way is an honor as well.”
Laboratories around the world face challenges in analyzing and making predictions from their data due to various reasons, but that’s where LabWare’s data analytics solutions come into play. LabWare created the Data Science Engine that enables laboratories to do analytics within the LabWare platform, enabling them to analyze their data efficiently and in new ways that impact decisions.
When the COVID pandemic changed the world, LabWare ensured organizations worldwide continued delivering life-saving diagnostics, therapies, and critical data. Mapping millions of COVID testing outcomes to geometric sampling data and patient demographics, LabWare provided advanced data trending to support the optimal locations of testing resources. LabWare embedded this type of data-driven approach in its informatics platform. Working closely with laboratories in many industries using the power of artificial intelligence, machine learning, and data science, LabWare brings them a competitive advantage by getting the most from their data.
“In today's Modern Lab, there’s a huge need to not only acquire data but also understand it and apply it to scientists' and lab manager’s tasks without taking them outside their normal work streams,” Michael Learner, Managing Director of LabWare North America, said. “That’s where LabWare Analytics comes in, to help our customers explore and implement the data
they’ve acquired. With our Data Science Engine, laboratories can identify patterns, extract insights, and gain a deeper understanding of their data to make informed decisions.”
Through client conversations, LabWare has found that having data science and machine learning foundational to their work enables their clients to succeed in the lab and beyond.
Topics: Analytics
3 min read
LabWare Rockets to the Top of G2 Spring 2023 Software Reports for LIMS, ELN, and Lab Inventory
By LabWare on Mar 31, 2023 11:58:33 AM
WILMINGTON, Del.-- LabWare, Inc., reinforcing it's position as the worldwide leader in enterprise laboratory automation software, today announced that it has been named "Leader" in the LIMS, ELN, and Lab Inventory Management Categories on G2's 2023 Reports.
Topics: LabWare
14 min read
Petrochemical LIMS Solutions: Enhancing Lab Efficiency
By LabWare on Jan 17, 2023 11:15:00 AM
Implementing robust LIMS (Laboratory Information Management System) is crucial for maintaining efficiency and accuracy in laboratory operations in the ever-evolving petrochemical industry. To ensure competitiveness and compliance, a configurable LIMS designed to meet the needs of the laboratories within the petrochemical industry will provide the necessary support for organizations.
Topics: Oil and Gas
15 min read
Compliance in High-throughput Petrochemical Testing Environments: Managing the Costs
By LabWare on Jan 5, 2023 1:14:38 PM
Complying with petrochemical testing regulations is a must for businesses in the industry, making sure that products meet quality specifications and regulatory needs. As such, it is essential for companies operating within this sector to stay informed about the latest advancements and best practices. This blog post will delve into various topics related to compliance in petrochemical testing.
Topics: Oil and Gas
12 min read
Optimizing Petrochemical Lab Management with LabWare LIMS
By LabWare on Nov 8, 2022 9:37:16 AM
In the rapidly evolving world of petrochemical lab management, staying ahead of industry trends and regulatory requirements is crucial for success. As a key player in this sector, it's essential to understand how effective laboratory practices can impact your organization's bottom line.
Topics: LIMS Oil and Gas
4 min read
How to Use Your Food Safety LIMS for Sensory Testing
By LabWare on Jul 7, 2022 8:15:00 AM
The sensory characteristics of food are directly tied to the overall food quality and can be the make-or-break feature concerning how consumers engage with your product. From testing and determining the food properties like appearance, flavor, and consistency, implementing adequate safety measures in your processes ensures your business remains compliant with regulatory standards while maintaining high quality.
Topics: Food and Beverage
4 min read
Yposkesi Implements LabWare’s Enterprise Laboratory Platform
By LabWare on Jul 2, 2022 7:42:59 AM
Photo: via Yposkesi
ALTRINCHAM, UK-- LabWare Limited
Yposkesi, an SK pharmteco company, is one of Europe’s largest Contract Development and Manufacturing Organizations (CDMO) for gene therapy viral vector manufacturing, which, after a formal selection process of the leading LIMS solutions within the market, chose to implement LabWare as their LIMS solution for their Corbeil-Essonnes (France) site.
Topics: LabWare QA/QC Environmental Monitoring
3 min read
LabWare Again Ranked LIMS Leader in G2 Summer 2022 Reports
By LabWare on Jun 30, 2022 10:07:39 AM
WILMINGTON, Del.-- LabWare, Inc., recognized worldwide as the leader in enterprise laboratory automation software, today announced that it has once again been ranked as a "Leader" in the LIMS Systems Category on G2's 2022 Summer Reports.
Topics: LabWare
4 min read
LabWare LIMS Supplies Tools and Workflows to Support These 5 Food & Beverage Industry Regulations
By LabWare on Jun 28, 2022 12:01:00 PM
Detailed criteria regulate production environments in the food and beverage industry. These regulations are in place to ensure that consumers are safe and that quality is consistent across product batches.
Topics: Food and Beverage
3 min read
How Chemistry Workflows Promote Food Safety
By LabWare on Jun 16, 2022 11:36:00 AM
In the food industry, safety testing is essential to providing high-quality products, preventing contamination and food-borne illness, and maintaining brand reputation. Food chemistry laboratories must control data from complex analyses while maintaining the highest analytical performance and regulatory standards to deliver on food safety. Meeting these standards can be arduous without a reliable laboratory information management system (LIMS).
Topics: Food and Beverage
14 min read
Microbiology Testing Food Safety: Advancing Lab Efficiency
By LabWare on Jun 7, 2022 10:30:00 AM
Microbiology testing is essential to guarantee consumer safety in the constantly changing field of food safety. This post will examine the significance of microbiological testing in food and beverage production, along with typical microorganisms tested to guarantee product security.
Topics: Food and Beverage
4 min read
Examples of LIMS Integrations at a Food Testing Lab
By LabWare on May 26, 2022 12:55:16 PM
Your food testing lab enables your business to continually keep running and satisfy your consumers. From adequately tracking samples, tests and results to conducting detailed oversight of your operational workflows, you must operate efficiently while ensuring that you comply with regulatory and good quality practices related to your products or services.
Topics: Food and Beverage
3 min read
How to Leverage Contract Testing for Your Food Industry Needs
By LabWare on Apr 19, 2022 9:03:00 AM
Finding an experienced and accredited third-party lab partner is vitally important in the food and beverage industry to assure consistently accurate results. There is a lot of competition, and there are many choices available to lab managers that may already be stretched thin by their job roles and duties.
The synergy between a third-party lab partner and its ability to impact revenue is crucial to nail down. The lab and the business influence each other and affect how the lab manager handles leveraging contract testing for food and beverage needs.
Topics: Food and Beverage
3 min read
How LIMS Prepares Your Food Testing Lab for a Quality Inspection
By LabWare on Apr 12, 2022 9:16:00 AM
As a food testing laboratory manager, managing your lab means ensuring efficient workflows with accurate data. In addition to practicing quality control (QC), you also have to carry out your processes in compliance with industry standards. Regulatory bodies like the Food and Drug Administration (FDA) and the Food Safety and Inspection Service (FSIS), and local agencies of countries to which you export may all have specific standards that you need to follow.
Topics: Food and Beverage
3 min read
Vital Traceability Tools for Food & Beverage Testing Labs
By LabWare on Mar 24, 2022 8:00:00 AM
The food supply chain is a system that comprises small and large domestic and foreign producers, processors, packers, distributors, and transporters with few common business practices. It is a system that is becoming more global: Fresh fruits and vegetables are now available year-round, as well as a wide array of exotic foods. These factors have increased the risk of a significant food safety incident.
Topics: Food and Beverage
12 min read
Efficient Food & Beverage QC Laboratory: Streamlining Processes with LIMS
By LabWare on Mar 17, 2022 7:45:00 AM
An efficient QC laboratory is crucial for maintaining quality and credibility in laboratory medicine. As the sector progresses, it is imperative to simplify activities, embrace cutting-edge technologies, and cultivate a spirit of cooperation inside clinical labs. In this blog post, we will delve into key strategies that can help you transform your lab into an efficient QC powerhouse.
Topics: Food and Beverage
4 min read
LabWare Holdings to Acquire Data Analytics Company CompassRed - Establishing LabWare Data Analytics Innovations Center
By LabWare on Feb 28, 2022 1:45:00 PM
WILMINGTON, Del.-- LabWare Holdings today announced it has signed a definitive agreement to acquire CompassRed, a visionary company in machine learning and predictive analytics. The acquisition of CompassRed will create a new dedicated advanced data analytics arm in LabWare®, embedding the CompassRed solution into the core LabWare platform, and providing services that will further elevate LabWare’s position as the global leader in the Laboratory Information Management market.
Topics: LabWare Analytics
5 min read
LIMS Mitigates Top Food Safety Risks and Drives Compliance
By LabWare on Jan 27, 2022 9:29:43 AM
Maintaining regulatory compliance is crucial to success in the food and beverage industry. Failure to follow guidelines may result in consequences, ranging from financial loss and operational downtime to complete shutdown.
Topics: Food and Beverage
15 min read
Optimizing Food Safety with LIMS HACCP Integration
By LabWare on Jan 10, 2022 8:00:00 AM
In the realm of food safety management, LIMS HACCP integration has emerged as a powerful tool for maintaining compliance and ensuring product quality. By combining the robust capabilities of modern Laboratory Information Management Systems (LIMS) with Hazard Analysis and Critical Control Points (HACCP) principles, organizations can streamline their hazard analysis processes and effectively manage critical control points.
Topics: LIMS Food and Beverage
4 min read
How LabWare Meets the Challenge of Managing an Enterprise LIMS
By LabWare on Sep 9, 2021 8:00:00 AM
UL is a trusted leader in safety science, providing a variety of certification, inspection, and testing services for businesses around the globe. Customers turn to UL to help them achieve safety, security, and sustainability goals in order to manage risk effectively and achieve regulatory compliance.
4 min read
Dutch Lab Expands the Functionality of LIMS for Inventory Management
By LabWare on Aug 26, 2021 8:00:00 AM
Note: This story was featured in the April 2021 edition of LABInsights and has been edited for clarity.
Denkavit recently expanded the functionality of its LIMS, adding options for inventory management, tracking consumption and ordering lab consumables. To facilitate these additions to an application the animal feed manufacturer has been using since 2014, Denkavit’s LIMS supplier adapted its existing inventory management template and added new, client-specific functionalities.
Topics: LabWare
13 min read
Streamlining Pharmaceutical Stability Testing with LabWare LIMS
By LabWare on Aug 13, 2021 7:45:00 AM
The evaluation of drug products' safety, efficacy and quality over time is an essential element of pharmaceutical stability testing. As the industry progresses, so do the strategies and technologies used for executing these essential studies. In this blog post, we will delve into various aspects of stability management within pharmaceutical testing.
15 min read
LIMS Stability Testing: Enhancing Efficiency in Labs
By LabWare on Aug 5, 2021 8:00:00 AM
In the realm of LIMS stability testing, efficient management and accurate analysis are critical for ensuring product quality and regulatory compliance. Traditional methods often fall short in providing a streamlined workflow, resulting in wasted resources and increased chances of errors. This blog post delves into the importance of an effective stability system, highlighting the limitations of conventional approaches while emphasizing the benefits offered by Laboratory Information Management Systems (LIMS).
Topics: Stability
5 min read
Pfizer COVID-19 Vaccine Manufacturing Process: How LIMS Plays a Key Role
By LabWare on Jul 21, 2021 8:00:00 AM
A recent New York Times article highlighted the manufacturing, testing, and distribution processes for the Pfizer-BioNTech Covid-19 messenger RNA (mRNA) based vaccine.
The careful execution, integration, and optimization of multiple steps are critical to the vaccine's safety, effectiveness, and efficient delivery. Pfizer's multifaceted vaccine process takes 60 days from start to finish across numerous locations involving thousands of employees. More than half of this effort goes to quality testing, which must adhere to vaccine manufacturing regulatory requirements, including compliance with current Good Manufacturing Practices (cGMP).
Topics: LabWare
14 min read
Cannabis Quality Control: Enhancing Testing via LIMS
By LabWare on Jun 17, 2021 8:00:00 AM
In the burgeoning cannabis industry, maintaining stringent cannabis quality control measures is of paramount importance to ensure consumer safety and regulatory compliance. As this sector continues to expand, businesses must adapt and implement advanced analytical testing methods that not only meet current standards but also anticipate future regulations.
Topics: LIMS Cannabis
14 min read
Optimizing Cannabis Testing Lab: Advanced LabWare Solutions
By LabWare on Jun 3, 2021 8:00:00 AM
As the cannabis industry continues to grow, the importance of reliable and accurate Cannabis Testing Labs cannot be overstated. The vital role of Cannabis Testing Labs in guaranteeing the safety and compliance of medical cannabis products cannot be underestimated. This post takes a look at the features of an advanced Cannabis Testing Lab, including its utilization of RFID barcodes for streamlined sample tracking and customer portal access to simplify ordering and remote access.
Topics: LIMS Cannabis
13 min read
Achieving Cannabis LIMS Compliance: A Comprehensive Guide
By LabWare on May 27, 2021 8:00:00 AM
In the rapidly growing cannabis industry, achieving and maintaining Cannabis LIMS Compliance is essential for specialized cannabis testing laboratories. As regulations evolve and ISO 17025 certification becomes increasingly important, labs must adapt to ensure they meet these stringent requirements.
Topics: LIMS Cannabis
14 min read
LIMS Environmental Monitoring: Boosting Lab Efficiency
By LabWare on May 18, 2021 8:00:00 AM
In the realm of LIMS environmental monitoring, implementing a robust and efficient system is crucial for ensuring quality control and compliance with regulatory requirements. As an advanced professional in this field, you understand the importance of streamlining lab workflows and automating processes to reduce errors and improve overall efficiency.
Topics: Environmental Monitoring
5 min read
The Benefits of Integrated Processes in Environmental Monitoring
By LabWare on Apr 22, 2021 7:45:00 AM
Laboratory operations are inherently expensive operations to develop and maintain, particularly in regulated industries. When operations are harmonized, the lab has the ability to leverage training, standard operating procedures (SOPs), instruments and infrastructure.
Topics: Environmental Monitoring
16 min read
High Volume Environmental Monitoring: LabWare LIMS Solutions
By LabWare on Apr 6, 2021 8:15:00 AM
High volume environmental monitoring is a crucial aspect of ensuring compliance with regulatory requirements and maintaining product quality standards. However, managing large-scale sample analysis presents unique challenges that can impact laboratory efficiency and data integrity. In this blog post, we will explore the various hurdles faced by organizations dealing with high sample volumes in their environmental matrices.
2 min read
LabWare Dominates Laboratory Software Category on G2 Grid Reports
By LabWare on Mar 24, 2021 12:35:56 PM
WILMINGTON, Del.-- LabWare, Inc., recognized worldwide as the leader in enterprise laboratory automation software, today announced that it has dominated the Laboratory Software Category in G2's 2021 Spring Grid Reports.
Topics: LabWare
15 min read
Overcoming Paperless Laboratory Risks: A Comprehensive Guide
By LabWare on Feb 25, 2021 7:30:00 AM
As laboratories consider transitioning to paperless systems, it is crucial to understand the potential risks associated with relying solely on electronic laboratory notebooks and digital data storage. In this blog post, we will delve into various aspects of paperless laboratory risks and discuss how they can be mitigated using advanced technology solutions.
Topics: Laboratory Management QA/QC
11 min read
LIMS Traceability Quality: Boosting Lab Performance
By LabWare on Feb 15, 2021 8:15:00 AM
Traceability and quality are indispensable in the realm of laboratory information management systems (LIMS), with a view to sustaining precision and effectiveness across multiple sectors. As technology continues to advance, it is essential for organizations to adopt innovative solutions that enhance their LIMS capabilities, particularly in terms of automating quality assurance processes, improving traceability through barcoding, and generating consistent certificates of analysis.
Topics: QA/QC
13 min read
Enhancing Cannabis Testing Laboratories with LIMS Solutions
By LabWare on Nov 5, 2020 8:00:00 AM
As the cannabis industry continues to expand, Cannabis Testing Laboratories play a crucial role in ensuring product quality and regulatory compliance. These labs are responsible for inspecting cannabis products to evaluate potency, check for contaminants and assess other components that affect their safety and effectiveness. In this blog post, we will delve into the importance of these laboratories for cultivators, manufacturers, and distributors alike.
Topics: LIMS Cannabis
13 min read
Achieving Successful LIMS Implementation in QC Laboratories
By LabWare on Oct 29, 2020 8:15:00 AM
As the demand for increased efficiency and accuracy in laboratories continues to grow, LIMS implementation has become a critical aspect of modern lab management. A successful LIMS implementation can revolutionize your laboratory's operations, streamlining workflows and ensuring data integrity. To ensure your laboratory operations are modernized and streamlined, this blog post will delve into the implementation of a Laboratory Information Management System (LIMS) that caters to your specific needs.
Topics: QA/QC
12 min read
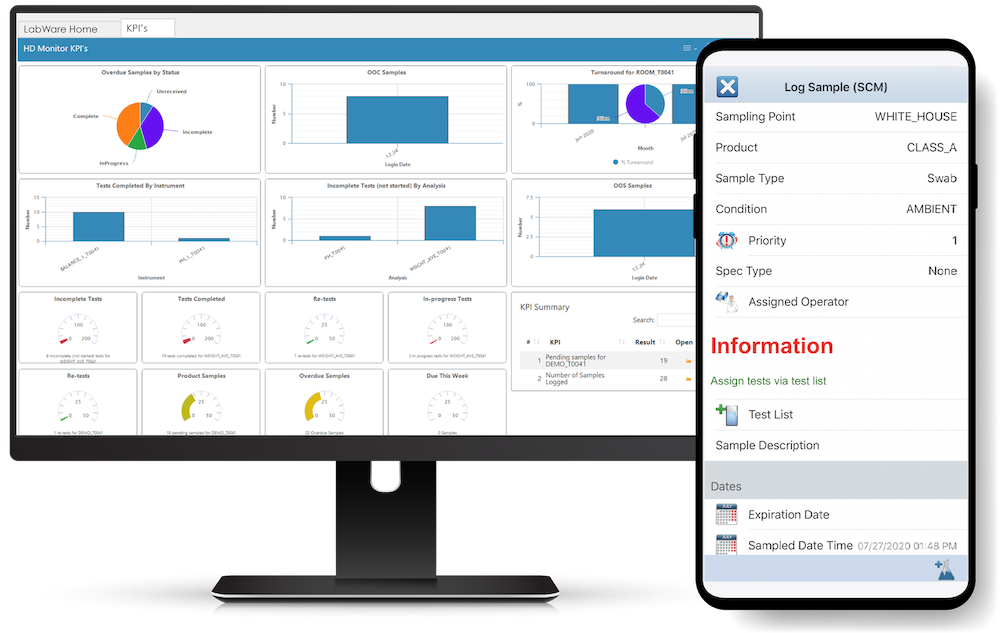
LIMS KPI Dashboards for Running an Efficient Lab
By LabWare on Sep 28, 2020 7:30:00 AM
Effective utilization of LIMS KPI Dashboards is crucial for driving laboratory efficiency and streamlining processes. In this blog post, we will delve into the importance of key performance indicators (KPIs) in a lab setting, focusing on how they enhance accountability and can be customized to cater to specific requirements.
Topics: LIMS LabWare
14 min read
Achieving Excellence with LIMS Quality Control
By LabWare on Sep 18, 2020 9:30:00 AM
Today, in the rapidly changing lab setting, quality control of LIMS is essential for attaining precise and dependable analytical data. A robust Laboratory Information Management System (LIMS) not only streamlines workflows but also enhances the overall quality management system within an organization.
Topics: LIMS QA/QC
6 min read
6 Best Practices for Effective QC Laboratory Management
By LabWare on Sep 11, 2020 12:00:00 PM
We spend a lot of time focusing on SOPs and setting up laboratory equipment and software in order to facilitate maximum compliance. Given the levels of technical details and specific procedures that are essential to proper lab functioning, it makes sense to build systems to effectively share this information and reinforce best practices.
But the culture in which a laboratory operates is critical as well, because it can drive how receptive the team is to adherence. Effective laboratory management relies on a few simple principles and tools that work to tip the scales into optimal lab function.
Topics: Laboratory Management
6 min read
Optimize Compounding Labs with Cloud-Based LIMS Solutions
By LabWare on Aug 18, 2020 8:15:00 AM
Cloud-Based LIMS Compounding is revolutionizing the pharmaceutical industry by providing an efficient and effective solution for managing laboratory data. As a cutting-edge technology, it offers numerous benefits that can help compounding pharmacies maintain regulatory compliance, improve quality control processes, and ensure sterilization consistency.
Topics: LIMS
14 min read
Maximize Lab Efficiency: Discover Cloud-Based LIMS
By LabWare on Aug 10, 2020 8:15:00 AM
As the life sciences industry continues to evolve, cloud-based LIMS (Laboratory Information Management Systems) have emerged as a powerful solution for managing complex laboratory data and processes. Cloud-based LIMS solutions provide a range of advantages over traditional on-premises systems, enabling organizations to optimize operations while meeting regulatory requirements.
Topics: LIMS
15 min read
LIMS Software Selection: Involving Stakeholders for Success
By LabWare on Jul 29, 2020 8:00:00 AM
Selecting the right LIMS (Laboratory Information Management System) software is a critical decision for any organization, as it directly impacts laboratory efficiency, data management, and regulatory compliance. In this blog post, we will discuss the importance of involving various stakeholders in the LIMS software selection process to ensure a comprehensive understanding of your organization's needs.
Topics: LIMS
13 min read
LIMS Market Growth: Cloud-Based Solutions Impact
By LabWare on Jul 15, 2020 8:00:00 AM
The LIMS market growth continues to gain momentum as laboratories worldwide seek advanced solutions for efficient data management and process automation. This surge in demand has sparked the development of cloud-based LIMS, which offer improved functionality compared to traditional self-hosted solutions.
Topics: LIMS
16 min read
Understanding LIMS Total Cost: Factors and Considerations
By LabWare on Jul 7, 2020 8:15:00 AM
Understanding the LIMS total cost is crucial for decision-makers when selecting a Laboratory Information Management System (LIMS) that best suits their organization's needs. This comprehensive guide will provide valuable insights into various aspects of LIMS, helping you make informed decisions about your investment.
Topics: LIMS
14 min read
7-Step Guide to LIMS Solutions Comparison
By LabWare on Jun 16, 2020 8:15:00 AM
As the demand for efficient laboratory management increases, a thorough comparison between LIMS solutions becomes crucial in selecting the best system to streamline your lab operations. To make an informed decision, this blog post will provide insights on how to compare different LIMS software.
Topics: LIMS
1 min read
LabWare® Announces LabWare 8
By LabWare on Jun 12, 2020 10:15:00 AM
WILMINGTON, Del.-- LabWare, Inc., recognized worldwide as the leader in enterprise laboratory automation software, today announced the production release of LabWare 8.
Topics: LabWare
15 min read
Navigating the LIMS Evaluation Process for Optimal Success
By LabWare on Jun 9, 2020 8:00:00 AM
The LIMS evaluation process is a key factor in achieving success, and this guide will provide invaluable guidance to ensure the right decisions are made. This guide will take you through the whole process of selecting a proper LIMS, while providing helpful advice to help choose the most appropriate provider.
Topics: LIMS
13 min read
Achieving LIMS Software Compliance: A Comprehensive Guide
By LabWare on Jun 4, 2020 8:00:00 AM
In the realm of LIMS software compliance, laboratory information management systems (LIMS) play a critical role in ensuring that laboratories adhere to regulatory guidelines and maintain data integrity. As the landscape of regulations continues to evolve, it is essential for organizations to understand and implement strategies for maintaining compliance within their LIMS environment.
Topics: LIMS
13 min read
Optimizing Efficiency with Remote Laboratory Solutions
By LabWare on May 18, 2020 11:53:31 AM
As the world continues to adapt to offsite work, Remote Laboratory Solutions have become increasingly important in maintaining efficient and secure operations. These solutions provide a myriad of benefits for laboratories, including streamlined workflows and improved collaboration among team members.
Topics: LIMS
15 min read
Achieving LIMS Audit Compliance with Lab Management Software
By LabWare on May 6, 2020 8:00:00 AM
In the world of laboratory management, LIMS audit compliance is a crucial aspect that can greatly impact an organization's success. With increasing regulatory focus on data integrity and electronic records, it is essential for laboratories to adopt a robust LIMS audit framework in order to maintain their credibility and avoid costly penalties.
Topics: LIMS
12 min read
Exploring SaaS LIMS Benefits for Modern Laboratories
By LabWare on May 1, 2020 8:15:00 AM
As businesses continue to evolve, SaaS LIMS benefits have become increasingly crucial for organizations seeking to optimize their laboratory operations. These cloud-based solutions offer a cost effective alternative to traditional LIMS software, providing numerous advantages that can transform the way labs function.
Topics: LIMS QA/QC
4 min read
8 Reasons Why Your Laboratory Needs a SaaS LIMS Solution
By LabWare on Apr 21, 2020 9:15:00 AM
There are hundreds or even thousands of moving parts and data points in a lab environment, making it vital to ensure you have the right tools in place to identify and place your resources. Gaining full visibility of your workflow has never been more important, particularly as compliance requirements evolve and require greater control and transparency throughout your lab.
Topics: LIMS
7 min read
15 Advantages of Using a SaaS LIMS Solution
By LabWare on Apr 16, 2020 8:00:00 AM
It's important to understand the advantages of a LIMS implementation and how it elevates the performance and efficiency of a laboratory. By automating adherence to best practices as well as introducing controlled sample and data management, laboratories can free up time and resources while also improving quality controls and laboratory performance measurement.
Topics: LIMS
11 min read
Optimizing Lab Efficiency with LIMS Workflow Management Solutions
By LabWare on Apr 8, 2020 8:00:00 AM
Efficient LIMS workflow management is essential for laboratories to streamline their processes, ensure data integrity, and maintain compliance with industry regulations. In this blog post, we will explore the various aspects of implementing an integrated LIMS solution that can help optimize your laboratory operations.
Topics: LIMS
15 min read
LabWare LIMS Comparison: Evaluating Key Features
By LabWare on Mar 24, 2020 5:35:04 PM
When it comes to LabWare LIMS, the sheer variety of features and capabilities offered by this industry-leading laboratory information management system (LIMS) are astounding. In this blog post, we will dive deep into some key aspects of LabWare LIMS that set it ahead of its competitors.